Phosphate rock (PR) is recommended for application to acid soils where phosphorus (P) is an important limiting nutrient on plant growth. The past 50 years have seen the accumulation of considerable knowledge regarding the factors affecting the agronomic effectiveness of PR for direct application. However, much less information is available on other effects associated with PR use, i.e. secondary nutrients, micronutrients, liming effect, and hazardous elements. This chapter presents a review of the information available in literature that is relevant to these other effects.
Among the significant chemical and nutritional constraints on crop growth on acid soils are deficiencies of calcium (Ca) and magnesium (Mg) nutrients. As the apatite mineral in PR is Ca-P, there is a potential to provide Ca nutrient if there are favourable conditions for apatite dissolution. Furthermore, many sources of PR contain free carbonates, such as calcite (CaCO3) and dolomite (CaMg(CO3)2), that can also provide Ca and Mg in acid soils. However, if dissolution of free carbonates raises pH and exchangeable Ca around PR particles significantly, it can hinder apatite dissolution and thus reduce P availability of PR (Chien and Menon, 1995b). For example, Chien (1977) found that Huila PR (Colombia), which contained about 10 percent CaCO3, increased soil solution pH from 4.8 to 6.2 in one week compared with other PRs that increased pH to 5.1. Consequently, the maximum soil solution P concentration obtained with Huila PR was lower than that obtained with central Florida PR (Figure 25), even though the two PR sources had approximately the same degree of isomorphic substitution of CO3 for PO4 in apatite structure.
Hellums et al. (1989) reported on the potential agronomic value of Ca in some PRs from South America and West Africa. Their study applied adequate P as KH2PO4 to an acid sandy loam (pH 4.5) with low exchangeable Ca to isolate the Ca from the P effect. The results showed that Ca uptake by maize with various PR sources followed the order of the reactivity of the PRs except Capinota PR (Bolivia), which had about 10 percent CaCO3 (Figure 26). The relative agronomic effectiveness (RAE) of various PR sources with respect to CaCO3 (100 percent) in terms of increasing dry-matter yield and Ca uptake ranged from 28 to 89 percent and from 8 to 58 percent, respectively (Table 26). The results showed that PRs of medium and high reactivity have potential Ca value, in addition to their use as a P source, when applied directly to acid soils with low exchangeable Ca.
In a three-year field trial conducted in central China, Hu et al. (1997) reported that exchangeable Ca increased from 1 194 mg/kg with the control to 1 300-2 100 mg/kg with PR treatments. The corresponding exchangeable Mg levels were 330 mg/kg with the control and 350-400 mg/kg with the PR treatments. Because the content of apatite-bound Mg is very small (unlike apatite-bound Ca), it is expected that PR will not increase soil exchangeable Mg significantly unless the PR contains a significant amount of dolomite. More research is needed to obtain information on the agronomic value of Ca and Mg (especially the latter).
FIGURE 25
Relationship between maximum P
concentration in soil solution and mole ratio of CO3:PO4
in the apatite structure

Source: Chien, 1977a.
FIGURE 26
Relationship between Ca uptake by maize
and citrate solubility of various PR sources
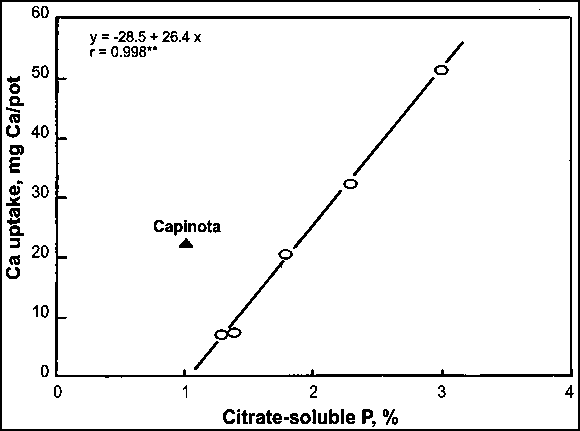
Source: Hellums et al., 1989.
TABLE 26
Relative agronomic effectiveness of
various PRs with respect to CaCO3 as a Ca source for
maize
|
Ca source |
Reactivity |
Relative agronomic effectiveness (%) |
|
|
Dry-matter yield |
Ca uptake |
||
|
Bahia Inglesa PR (Chile) |
High |
89 |
58 |
|
Bayovar PR (Peru) |
High |
73 |
33 |
|
Capinota PR (Bolivia) |
Low |
52 |
17 |
|
Tilemsi Valley PR (Mali) |
Medium |
53 |
17 |
|
Tahoua PR (Niger) |
Low |
31 |
8 |
|
Hahotoe PR (Togo) |
Low |
28 |
8 |
|
CaCO3 |
|
100 |
100 |
Source: Hellums et al., 1989.
Some PR sources may contain a significant amount of sulphur (S) bearing accessory minerals, e.g. gypsum (CaSO4) in Israeli PR (Axelrod and Gredinger, 1979) and pyrite (FeS2) and pyrrhotite (FeS) in Mussoorie PR, India (PPCL, 1983). However, little information is available on S availability to plants from these PR sources.
Some PRs contain accessory minerals that may provide micronutrients to aid plant growth. However, limited information is available on this potential additional benefit of using PR for direct application.
Work by Hammond et al. (1986b) on an Oxisol in Colombia suggested that indigenous Huila PR, which contains 136 mg of zinc (Zn) per kilogram, produced a higher grain yield of one rice variety (Cica-8) than did triple superphosphate (TSP) because of its Zn content (Figure 27). However, available Zn from Huila PR alone was not sufficient to provide adequate Zn for two rice varieties. When Zn was applied to the soil, both Huila PR and TSP were equally effective in increasing rice grain yield.
In New Zealand, Sinclair et al. (1990) found that Sechura PR (Peru), which contains 43 mg of molybdenum (Mo) per kilogram, increased dry-matter yields of pasture herbage more than TSP did at sites where the PR increased Mo levels in clover significantly (Figure 28). More information is needed on the micronutrient contents of PRs that have potential for increasing crop production on acid soils.
FIGURE 27
Response of rainfed rice to huila PR
and TSP on an Oxisol

Source: Hammond et al., 1986b.
FIGURE 28
Effect of Sechura PR and TSP on Mo
concentration in clover

Source: Sinclair et al., 1990.
Low pH coupled with toxic levels of aluminium (Al) and manganese (Mn) frequently contributes to poor soil fertility for plant growth on acid tropical and subtropical soils in developing countries. Although lime is effective in alleviating soil acidity and Al toxicity, it is often either unavailable or expensive to transport. Screening crop species and varieties to identify those that are tolerant of soil acidity would reduce lime requirements (Sanchez and Salinas, 1981; Goedert, 1983).
The dissolution of apatite in PR consumes H+ ions and, thus, it can increase soil pH, depending on PR reactivity. If a PR contains a significant amount of free carbonates, it can further increase soil pH. However, although an increase in soil pH may reduce the Al saturation level, it can also reduce apatite dissolution at the same time. The optimum condition would call for a soil pH that is high enough to reduce the Al saturation level but still low enough for apatite dissolution to release P.
Research by the International Fertilizer Development Center (IFDC) has shown that the application of medium to highly reactive PRs with low free-carbonate contents can result in significant liming effects on acid soils. Although the increase in pH is generally less than 0.5 units, the decrease in exchangeable Al can be significant where the soil pH is less than 5.5 (Chien and Friesen, 2000) as the exchangeable Al level would be almost zero at this soil pH in Oxisols and Ultisols (Pearson, 1975). For example, exchangeable Al was reduced from 2.0 to 0.4 meq/100 g when a Colombian Oxisol was treated with Sechura PR in a soil incubation study (Figure 29). The soil pH increased correspondingly from 4.6 to 5.0, and the exchangeable Ca rose from 0.2 to 1.5 meq/100 g. Consequently, the Al saturation level also declined from about 80 to 20 percent. Thus, the better performance of highly reactive PRs, e.g. Sechura and North Carolina, compare with TSP in plant-growth response on the soil may have been related to the alleviation of Al toxicity (Figure 30). In a five-year field trial conducted in an Oxisol fertilized with various PR sources, Chien et al. (1987b) reported that the pH increased from 4.1 with the control to 4.7-5.0 with the PR treatments. The corresponding increase in exchangeable Ca was from 0.17 cmol/kg with the control to 0.31-0.56 cmol/kg with the PR treatments. However, no significant effect on exchangeable Al was observed. In their study with the red soil of China, Hu et al. (1997) reported that the soil pH increased from 4.8 with the control to 4.9-5.3 with the PR treatments. A reduction in exchangeable Al of up to 70 percent with respect to the control was also observed with the PR treatments. Thus, the studies suggest that the application of PR to acid soils can also improve soil properties as well as the supply of available P for crop production.
FIGURE 29
Exchangeable Al and Ca in an Oxisol
treated with PRs and TSP at 200 mg P/kg during incubation
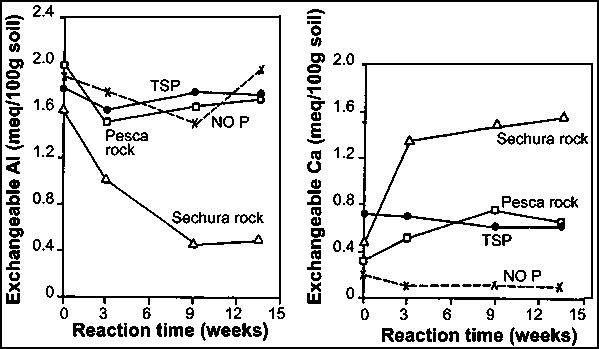
Source: Chien, 1982.
FIGURE 30
Effect of P sources on Panicum
maximum dry-matter yield (sum of three cuts) on an
Oxisol
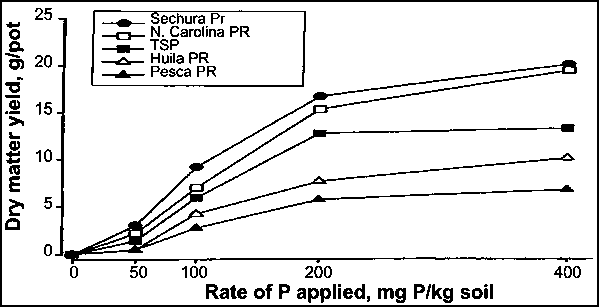
Source: Chien, 1982.
Sikora (2002) conducted a theoretical and experimental study to calculate and quantify the liming potential of PRs by laboratory titration and soil incubation. Of the three anions (PO4-3, CO3-2 and F-) present in the carbonate apatite structure of PR, CO3-2 and PO4-3 can consume H+ and cause an increase in pH. Because of the greater molar quantity of PO4-3 compared with CO3-2, PO4-3 exerts a greater effect on the liming potential of PR. The results for the titration of two PRs (highly reactive North Carolina and low-reactive Idaho) showed the ranges of calcium carbonate equivalence (CCE) were from 39.9 to 53.7 percent, which were less than the theoretical values (59.5 to 62.0 percent). The experimental model obtained from the soil incubation study showed qualitative agreement with theory as it showed increased liming ability with increased dissolved P from the PRs. However, the model showed lower percentage CCEs than theoretical calculations when the P dissolved ranged from 20 to 60 percent. Further research is needed to compare actual percentage CCE models across a variety of soil types in order to assess the potential liming effect associated with PR use.
All PRs contain hazardous elements including heavy metals, e.g. cadmium (Cd), chromium (Cr), mercury (Hg) and lead (Pb), and radioactive elements, e.g. uranium (U), that are considered to be toxic to human and animal health (Mortvedt and Sikora, 1992; Kpomblekou and Tabatabai, 1994b). The amounts of these hazardous elements vary widely among PR sources and even in the same deposit. Table 27 shows the results of a chemical analysis of potentially hazardous elements in some sedimentary PR samples (Van Kauwenbergh, 1997).
Among the hazardous heavy metals in P fertilizers, Cd is probably the most researched element. This is because of its potentially high toxicity to human health from consuming foods that are derived from crops fertilized with P fertilizers containing a significant amount of Cd. Most of the studies on Cd uptake by crops have used water-soluble P fertilizers such as TSP, single superphosphate (SSP), di-ammonium phosphate and mono-ammonium phosphate. However, the Cd reaction with soil treated with PR differs significantly from that with water-soluble P fertilizers because apatite-bound Cd in PR is water insoluble. Iretskaya et al. (1998) reported highly reactive North Carolina PR containing 47 mg of Cd per kilogram was as effective as SSP produced from the same PR in increasing grain yield of upland rice, but that the Cd concentration in rice grain with the PR was only about half of that with SSP. Thus, the information on Cd availability from water-soluble P sources cannot be implied directly to PR application.
TABLE 27
Chemical analysis of potentially
hazardous elements in sedimentary phosphate rocks
|
Country |
Deposit |
Reactivity |
P2O5 |
As |
Cd |
Cr |
Pb |
Se |
Hg |
U |
V |
|
Algeria |
Djebel Onk |
High |
29.3 |
6 |
13 |
174 |
3 |
3 |
61 |
25 |
41 |
|
Burkina Faso |
Kodjari |
Low |
25.4 |
6 |
<2 |
29 |
<2 |
2 |
90 |
84 |
63 |
|
China |
Kaiyang |
Low |
35.9 |
9 |
<2 |
18 |
6 |
2 |
209 |
31 |
8 |
|
India |
Mussoorie |
Low |
25.0 |
79 |
8 |
56 |
25 |
5 |
1 672 |
26 |
117 |
|
Jordan |
El Hassa |
Medium |
31.7 |
5 |
4 |
127 |
2 |
3 |
48 |
54 |
81 |
|
Mali |
Tilemsi |
Medium |
28.8 |
11 |
8 |
23 |
20 |
5 |
20 |
123 |
52 |
|
Morocco |
Khouribga |
Medium |
33.4 |
13 |
3 |
188 |
2 |
4 |
566 |
82 |
106 |
|
Niger |
Parc W |
Low |
33.5 |
4 |
<2 |
49 |
8 |
<2 |
99 |
65 |
6 |
|
Peru |
Sechura |
High |
29.3 |
30 |
11 |
128 |
8 |
5 |
118 |
47 |
54 |
|
Senegal |
Taiba |
Low |
36.9 |
4 |
87 |
140 |
2 |
5 |
270 |
64 |
237 |
|
Syrian Arab Republic |
Khneifiss |
Medium |
31.9 |
4 |
3 |
105 |
3 |
5 |
28 |
75 |
140 |
|
United Republic of Tanzania |
Minjingu |
High |
28.6 |
8 |
1 |
16 |
2 |
3 |
40 |
390 |
42 |
|
Togo |
Hahotoe |
Low |
36.5 |
14 |
48 |
101 |
8 |
5 |
129 |
77 |
60 |
|
Tunisia |
Gafsa |
High |
29.2 |
5 |
34 |
144 |
4 |
9 |
144 |
12 |
27 |
|
United States of America |
Central Florida |
Medium |
31.0 |
6 |
6 |
37 |
9 |
3 |
371 |
59 |
63 |
|
United States of America |
North Carolina |
High |
29.9 |
13 |
33 |
129 |
3 |
5 |
146 |
41 |
19 |
|
Venezuela |
Riecito |
Low |
27.9 |
4 |
4 |
33 |
<2 |
2 |
60 |
51 |
32 |
Source: Van Kauwenbergh, 1997.
The reactivity of the PR influences the availability of Cd to the plant because Cd is bound with P in the apatite structure (Sery and Greaves, 1996). To separate the P effect on Cd availability from PR, Iretskaya et al. (1998) pretreated two acid soils with 200 mg of P per kilogram as KH2PO4 so that no P response from PR would be expected in increasing grain yield of upland rice. They found that total Cd uptake by rice from the low-reactive Togo PR was 80 percent of that from the highly reactive North Carolina PR in the soil with a pH of 5.0, and 52 percent in the soil with a pH of 5.6 when the soils were treated with 400 µg of Cd per kilogram from the two PRs. McLaughlin et al. (1997) found that Cd concentrations in clover grown on the soil treated with a lower reactive Hamrawein PR (Egypt) containing 5.3 mg of Cd per kilogram were lower than that treated with highly reactive North Carolina PR containing 40.3 mg of Cd per kilogram at the same P rates. Thus, a PR source with a higher reactivity and Cd content can release more Cd than a PR with a lower reactivity and/or low Cd content for plant uptake. In addition to PR reactivity and Cd content, plant uptake of Cd also depends on soil properties, especially soil pH, and crop species (Iretskaya and Chien, 1999). More research is needed to investigate their interactions and integrate these factors on Cd availability associated with PR use.
Some PR sources may contain a significant amount of radioactive elements compared with other PR sources, e.g. 390 mg of U per kilogram in Minjingu PR (the United Republic of Tanzania) versus 12 mg of U per kilogram in Gafsa PR (Tunisia) (Table 27). As Minjingu PR is highly reactive and agronomically and economically suitable for direct application to acid soils for crop production (Jama et al., 1997; Weil, 2000), there has been concern over the safety of using this PR. Samples of soil and plant tissue associated with the use of this PR were collected by the International Centre for Research in Agroforestry and sent to the International Atomic Energy Agency for radioactivity testing. The results showed that the radioactivity of the soil and plant samples was about the same as the background levels. However, the potential safety problem remains a concern for workers during mining operations.
Most PRs also have high concentrations of fluorine (F) in apatite minerals, often exceeding 3 percent by weight (250 g of F per kilogram of P). Excessive F absorption has been implicated in causing injury to grazing stock through fluorosis. McLaughlin et al. (1997) reported no significant differences between F in herbage from plots fertilized with either SSP containing 1.7 percent F or North Carolina PR containing 3.5 percent F, or between sites that had received both fertilizers. Concentrations of F in herbage were generally less than 10 mg of F per kilogram and often near the detection limit for the analysis technique (1 mg of F per kilogram). They concluded that plant uptake of F is unlikely to lead to problems for grazing animals in most soils. However, they cautioned that ingestion of soil by animals or ingestion of fertilizer material remaining on herbage after heavy topdressing could affect animal health, depending on soil and fertilizer F concentrations. Thus, there is a need to manage F in PRs in long-term applications to acid soils.