Tekalign Mamo¹, I. Haque and C.S. Kamara²Soils and Plant Nutrition Section International Livestock Centre for Africa (ILCA)
PO Box 5689, Addis Ababa, Ethiopia
1. Present address: Agricultural Research Center, PO Box 32, Debre Zeit, Ethiopia2. On leave from Soil Science Department, Njala University College, Sierra Leone
Abstract
Introduction
Materials and methods
Results and discussion
Summary and conclusions
References
The phosphorus status of some Ethiopian highland Vertisols (15 surface and 37 profile samples) was investigated by determining P sorption capacity, P fractions and available P. Generally, the soils exhibited some differences in their P status. The content of organic P decreased within the soil profiles similar to the organic matter content, while the distribution of the other P fractions within the soil profiles had no consistent trend. Available P was generally limited, reflecting the low content of the active P forms (Ca-P, Fe-P and Al-P) in the soil profiles.
Vertisols cover 10.3% (about 12.7 million ha) of the Ethiopian land mass and are the fourth most abundant soils after Histosols, Cambisols and Nitosols. Vertisols may be found in the 0-8% slope range, but are more abundant in the 0-2% range (Berhanu Debele, 1985).
It is estimated that Vertisols comprise about 24% of all cropped highland soils (Jutzi and Haque, 1985). Vertisols are potentially among the most productive soils of sub-Saharan Africa, but they are agriculturally underutilised within the traditional farming practices due to excess soil moisture from waterlogging during the heavy rains.
Nitrogen and P are the two most important elements which are relatively low in Vertisols (Dudal, 1965; Hubble, 1984). With P. the problem is more of unavailability than of total quantity present in the soil.
The Ethiopian soils, similar to the other agricultural soils of the tropics, are generally low in N and P. Several authors have reported independently that 70-75% of some Ethiopian agricultural soils are deficient in P (Desta Beyene, 1982; Pulschen, 1987; Tekalign and Haque, unpublished data 2). However, very little detailed work has been done on the P status of Ethiopian soils and most of the studies on these soils have been concerned with crop productivity. The characterisation and the distribution of the different chemical forms of P have received little attention. This study presents the results of investigations on the relative distribution of the various P forms, P fixing capacity and available P status of some Ethiopian highland Vertisols.
Soil sampling analysis
Fifteen soil samples from the plough layer and 37 soil profile samples were collected from various sites (see Table 1). Soil samples were air dried in the laboratory, crushed, passed through a 2-mm sieve and stored for physico-chemical analysis. Particle size analysis was carried out by the method of Bouyoucos (1951). Organic matter was determined by the method of Walkley and Black (1934), and pH measured in 1:1 soil: water and 1:2.5 soil: CaCl2 ratios. Extractable Fe and Al were determined by the method of Mehra and Jackson (1960) and the contents read on an atomic absorption spectrophotometer.
Table 1. Sampling locations and site characteristics.
|
Soil No. |
Location |
Longitude |
Latitude |
Altitude |
|
1614 |
Debre Zeit (ILCA substation) |
38° 58' |
8° 44' |
1830 |
|
1615 |
Shola (ILCA station) |
38° 45' |
9° 00' |
2380 |
|
2295 |
Suke (ESCRP site)a |
40° 59' |
9° 07' |
1980 |
|
2297 |
Tis Abay Falls |
37° 35' |
11° 29' |
1600 |
|
2298 |
Fogera Plains |
37° 25' |
13° 36' |
1802 |
|
2299 |
Debre Birhan (ILCA substation) |
39° 38' |
9° 36' |
2780 |
|
2301 |
Debre Birhan (ILCA substation)b |
39° 38' |
9° 36' |
2780 |
|
2307 |
Robe |
39° 52' |
7° 38' |
1700 |
|
3669 |
Weretac |
37° 10' |
10° 50' |
1800 |
|
3672 |
Weretac |
37° 10' |
10° 50' |
1800 |
|
3732 |
Enewaric |
39° 15' |
9° 40' |
2600 |
|
3749 |
Wereiluc |
39° 31' |
10° 36' |
2600 |
|
3753 |
Wereiluc |
39° 31' |
10° 36' |
2600 |
|
4454 |
Wejelc |
38° 00' |
10° 00' |
1800 |
|
4059 |
Mega/Sidamo (ILCA Rangelands) |
38° 18' |
4° 03' |
2215 |
a. ESCRP = Ethiopian Soil Conservation Research Project.
b. Vertisol with overlying colluvial deposit.
c. ILCA Vertisols Project site.
Phosphorus estimation
Total P was determined by HClO4 digestion (Jackson, 1964) and organic P was estimated by the difference between extractable inorganic P before and after ignition by the method of Legg and Black (1955). Inorganic P was fractionated by the method of Chang and Jackson (1957) as modified by Peterson and Corey (1966). Available P was estimated by extraction with acid fluoride (Bray and Kurtz, 1945), and by Olsen's NaHCO3 method (Olsen et al, 1954). Phosphorus in all extracts was determined calorimetrically by the molybdenum blue colour method of Murphy and Riley (1962). Phosphorus sorption was studied using the method of Fox and Kamprath (1970).
Physico-chemical properties
The data in Table 2 show the general properties of the surface (0 to 15 em) soils used in this study. As would be expected, all the soils except one were of clay texture, containing an average of 62.6% clay. Soil 2301 had the lowest clay content since it represents a buried Vertisol with overlying colluvial deposits. The pH (in soil: water) of the Vertisols varied between 4.80 and 7.72 with a mean value of 5.88. Organic matter and total N contents were also within the ranges reported by earlier workers (Kamara and Haque, 1987).
Table 2. Some physico-chemical characteristics of the soils studied.
|
Soil No. |
Sand (%) |
Silt (%) |
Clay (%) |
Organic matter (%) |
Total N (%) |
pH (1:2.5) |
DCBa Extractable |
||
|
H2O |
CaCl2 |
Fe |
Al |
||||||
|
1614 |
26 |
15 |
59 |
2.35 |
0.10 |
6.89 |
6.06 |
9.3 |
0.8 |
|
1615 |
24 |
14 |
62 |
3.69 |
0.12 |
6.30 |
6.28 |
13.9 |
1.8 |
|
2295 |
22 |
14 |
64 |
3.29 |
0.14 |
4.80 |
4.25 |
22.5 |
0.3 |
|
2297 |
16 |
10 |
74 |
2.08 |
0.09 |
6.10 |
5.60 |
20.1 |
1.3 |
|
2298 |
18 |
16 |
66 |
4.39 |
0.21 |
5.85 |
5.50 |
37.4 |
2.6 |
|
2299 |
19 |
19 |
62 |
6.73 |
0.33 |
5.10 |
4.40 |
18.2 |
5.3 |
|
2301 |
37 |
26 |
37 |
4.65 |
0.21 |
5.40 |
5.15 |
18.1 |
3.8 |
|
2307 |
24 |
29 |
46 |
8.64 |
0.23 |
5.60 |
5.25 |
9.5 |
0.3 |
|
3669 |
18 |
17 |
65 |
1.75 |
0.09 |
6.05 |
5.03 |
38.8 |
1.4 |
|
3672 |
18 |
21 |
61 |
2.56 |
0.15 |
5.22 |
4.17 |
9.6 |
0.4 |
|
3732 |
20 |
19 |
61 |
2.87 |
0.11 |
5.89 |
4.93 |
7.7 |
0.4 |
|
3749 |
15 |
21 |
64 |
2.48 |
0.13 |
5.18 |
4.10 |
7.2 |
0.5 |
|
3753 |
19 |
21 |
60 |
1.50 |
0.06 |
6.22 |
4.89 |
8.5 |
0.3 |
|
4059 |
24 |
8 |
66 |
2.97 |
0.11 |
7.72 |
6.59 |
2.9 |
0.8 |
|
4454 |
15 |
21 |
64 |
3.39 |
0.15 |
5.40 |
4.44 |
8.3 |
0.2 |
|
Meanb |
19.9 |
17.5 |
62.6 |
3.48 |
0.14 |
5.88 |
5.11 |
15.3 |
1.2 |
a. Dithionite-citrate-bicarbonate.
b. Excludes soil 2301.
The distribution within the soil profiles of the physical and chemical properties for eight of the Vertisols is shown in Table 3. The percentage clay of sometimes increased with depth, sometimes decreased, and sometimes did not change significantly below the surface horizon. The variation seems to be the result of differences in the weathering of the parent materials and soil forming processes. The pH of the Vertisols increased with depth except in soil 4059 where a slight decrease was noted. Similar trends were also reviewed by Ahmad (1986) and Dudal (1965). Total N (Table 3) and organic matter (Figure 1) contents also followed a decreasing pattern with increasing profile depth. The C:N ratios for the surface soils varied between 7.7 and 11.8 and the trend was variable with depth.
Total phosphorus
Data on total P and other forms of P are presented in Tables 4 and 5. From Table 4, it is clear that soil 3732 contains the minimum amount of total P at the surface. The mean total P content for the surface samples of the 15 Vertisols is 453 ppm. The majority of the surface samples had values greater than 200 ppm which is the value indicated by Olsen and Engelstad (1972) as the maximum total P value for highly weathered tropical soils. On the other hand, the values are of the same order of magnitude as those in other tropical soils of lesser degree of weathering.
The lowest profile total P was observed in soil 1615 (Table 5) which also has the lowest available P (Bray II and Olsen) (Table 4).
Soil samples from profile 1615 and 4059 have profile total P values similar to the values reported by Piccolo and Gobena Huluka (1986) as the profile average of two Ethiopian Vertisols from Awasa (170 ppm) and Ginchi (200 ppm).
Table 3. Distribution of clay, pH, N and P in the soil profiles of some of the Vertisols.
|
Soil No. |
Depth (cm) |
pH (1:2.5) |
Clay (%) |
N (%) |
C:N |
C:P |
Avail. P Bray II (ppm) |
|
|
H2O |
CaCl2 |
|||||||
|
|
0-25 |
6.27 |
5.20 |
59 |
0.08 |
10.8 |
66.5 |
18.1 |
|
|
25-39 |
6.37 |
5.29 |
53 |
0.07 |
11.7 |
74.2 |
11.2 |
|
|
39-60 |
6.59 |
5.55 |
49 |
0.06 |
13.5 |
90.2 |
9.3 |
|
1614 |
60-112 |
7.31 |
6.01 |
54 |
0.05 |
10.5 |
65.6 |
0.1 |
|
|
112-167 |
7.75 |
6.34 |
24 |
0.02 |
11.6 |
58.1 |
39.8 |
|
|
167-192 |
8.03 |
6.38 |
17 |
0.01 |
9.2 |
30.7 |
124.1 |
|
|
192-205 |
8.21 |
6.32 |
6 |
0.01 |
9.0 |
30.7 |
191.2 |
|
|
0-23 |
5.52 |
4.48 |
60 |
0.21 |
11.0 |
202.9 |
0.3 |
|
|
23-100 |
5.63 |
4.65 |
66 |
0.07 |
10.3 |
119.9 |
0.1 |
|
1615 |
100-127 |
6.05 |
4.88 |
72 |
0.03 |
12.0 |
71.9 |
5.5 |
|
|
127-164 |
6.34 |
5.16 |
74 |
0.01 |
9.6 |
32.2 |
7.5 |
|
|
164-200 |
6.43 |
5.29 |
74 |
0.01 |
7.9 |
39.5 |
12.9 |
|
|
0-60 |
4.98 |
3.92 |
52 |
0.25 |
9.6 |
50.9 |
0.8 |
|
2299 |
60-140 |
5.90 |
4.38 |
60 |
0.10 |
12.8 |
67.2 |
0.5 |
|
|
140-170 |
6.58 |
4.88 |
54 |
0.07 |
9.8 |
45.9 |
35.0 |
|
|
170-200 |
6.38 |
4.66 |
52 |
0.01 |
0.9 |
3.7 |
49.7 |
|
|
0-20 |
5.95 |
4.25 |
28 |
0.27 |
7.7 |
83.5 |
1.2 |
|
|
20-38 |
5.85 |
4.40 |
44 |
0.13 |
14.1 |
98.3 |
0.04 |
|
2301 |
38-67 |
6.14 |
5.10 |
48 |
0.09 |
5.8 |
59.1 |
60.3 |
|
|
67-200 |
6.23 |
4.70 |
20 |
0.01 |
3.1 |
17.1 |
3.3 |
|
|
0-78 |
5.89 |
4.93 |
62 |
0.11 |
11.4 |
103.2 |
0.6 |
|
|
78-105 |
6.12 |
5.05 |
62 |
0.12 |
10.5 |
75.8 |
1.4 |
|
3732 |
105-128 |
7.48 |
6.52 |
30 |
0.03 |
8.8 |
49.7 |
9.6 |
|
|
128-200 |
7.43 |
6.37 |
30 |
0.01 |
3.0 |
10.6 |
55.0 |
|
|
0-34 |
5.18 |
4.10 |
64 |
0.13 |
8.4 |
49.4 |
0.8 |
|
|
34-80 |
5.35 |
4.25 |
66 |
0.09 |
9.0 |
50.7 |
0.2 |
|
3749 |
80-115 |
6.00 |
4.87 |
68 |
0.08 |
8.4 |
60.9 |
0.1 |
|
|
115-200 |
6.29 |
5.02 |
48 |
0.03 |
9.1 |
52.3 |
1.5 |
|
|
0-50 |
7.72 |
6.59 |
67 |
0.11 |
11.8 |
128.9 |
3.6 |
|
|
50-90 |
7.74 |
6.49 |
69 |
0.09 |
13.1 |
159.4 |
4.2 |
|
4059 |
90-115 |
7.51 |
6.53 |
71 |
0.07 |
13.9 |
152.1 |
16.8 |
|
|
115-175 |
7.34 |
6.54 |
67 |
0.02 |
8.8 |
92.3 |
20.6 |
|
|
175-200 |
7.26 |
6.48 |
75 |
0.02 |
5.5 |
78.3 |
10.5 |
|
|
0-40 |
5.42 |
4.46 |
64 |
0.15 |
9.9 |
74.3 |
2.6 |
|
|
40-95 |
5.30 |
4.32 |
72 |
0.10 |
9.4 |
46.0 |
0.1 |
|
4454 |
95-152 |
5.21 |
4.22 |
74 |
0.09 |
8.8 |
62.5 |
2.0 |
|
|
152-200 |
6.39 |
5.49 |
74 |
0.05 |
10.4 |
65.5 |
192.3 |
Figure 1. Distribution of organic matter within the soil profiles of some of the Vertisols.

From on-going studies on some Ethiopian soils, Tekalign and Haque (unpublished data 1), found that soils derived from basaltic rocks/volcanic materials contained the highest amounts of total P. For example, a total P content of 1981 ppm was found in a volcanic ash soil from Debre Sina. This soil also had high contents of Fe2O3 and Al2O3, which have a high capacity to occlude P (Chang and Jackson, 1957), although an additional suitable explanation could be found from the contribution to total P of the high quantity of organic P present in the same soil. According to the review by Ahmad (1986), total P contents of Vertisols derived from basic rocks/volcanic materials in the USA, India and the Caribbean are only about 50% of those of comparable Ethiopian soils.
Table 4. Available, fixed, organic, total and inorganic P fraction values (ppm) in the surface soils of the Vertisols.
|
Soil No. |
Available P |
Fixed P |
Organic P |
Total P |
Inorganic P fractions |
||||
|
Bray I |
Bray II |
Olsen |
Al-P |
Fe-P |
Ca-P |
||||
|
1614 |
3.1 |
21.5 |
5.8 |
105 |
124 |
368 |
12 |
27 |
98 |
|
1615 |
0.4 |
0.8 |
0.1 |
240 |
109 |
185 |
4 |
25 |
14 |
|
2295 |
0.7 |
17.3 |
3.6 |
220 |
320 |
818 |
11 |
48 |
260 |
|
2297 |
0.5 |
3.4 |
2.4 |
245 |
141 |
322 |
7 |
54 |
17 |
|
2298 |
1.5 |
4.0 |
21.5 |
400 |
367 |
981 |
24 |
234 |
54 |
|
2299 |
1.0 |
3.1 |
5.4 |
600 |
270 |
610 |
14 |
165 |
53 |
|
2301 |
6.1 |
12.2 |
18.5 |
125 |
299 |
640 |
60 |
145 |
40 |
|
2307 |
1.3 |
2.7 |
2.2 |
220 |
266 |
415 |
7 |
88 |
11 |
|
3669 |
1.6 |
34.7 |
25.3 |
385 |
356 |
311 |
15 |
138 |
44 |
|
3672 |
1.4 |
28.8 |
4.1 |
198 |
171 |
767 |
14 |
46 |
16 |
|
3732 |
6.8 |
37.3 |
14.6 |
112 |
130 |
141 |
15 |
25 |
23 |
|
3749 |
1.0 |
23.8 |
10.9 |
210 |
179 |
350 |
12 |
40 |
18 |
|
3753 |
0.3 |
23.3 |
5.1 |
180 |
99 |
326 |
15 |
18 |
20 |
|
4059 |
0.2 |
93.9 |
24.1 |
87 |
103 |
196 |
19 |
23 |
37 |
|
4454 |
3.8 |
70.9 |
22.1 |
178 |
291 |
376 |
26 |
66 |
37 |
Table 4. Total P values and the percentage distribution of active P forms in some of the profiles.
|
Soil No. |
Depth (cm) |
Total P (ppm) |
Al-Pa (%) |
Fe-Pa (%) |
Ca-Pa (%) |
|
|
0-25 |
359 |
9.4 |
12.5 |
78.1 |
|
|
25-39 |
524 |
8.2 |
5.1 |
86.7 |
|
|
39-60 |
443 |
13.9 |
36.0 |
51.8 |
|
1614 |
60-112 |
327 |
11.4 |
11.4 |
77.3 |
|
|
112-167 |
708 |
0.9 |
12.1 |
87.0 |
|
|
167-192 |
735 |
0.8 |
4.6 |
94.6 |
|
|
192-205 |
795 |
0.5 |
2.6 |
96.9 |
|
|
0-23 |
161 |
14.1 |
70.5 |
15.4 |
|
|
23-100 |
76 |
4.8 |
85.5 |
9.6 |
|
1615 |
100-127 |
123 |
2.5 |
56.8 |
40.7 |
|
|
127-164 |
110 |
2.6 |
62.1 |
35.3 |
|
|
164-200 |
104 |
9.9 |
12.7 |
77.5 |
|
|
0-60 |
972 |
13.4 |
51.3 |
35.3 |
|
2299 |
60-140 |
365 |
19.2 |
47.7 |
33.3 |
|
|
140-170 |
561 |
93.6 |
69.2 |
57.7 |
|
|
170-200 |
338 |
29.4 |
11.8 |
58.8 |
|
|
0-20 |
493 |
4.9 |
74.3 |
20.8 |
|
|
20-38 |
250 |
12.2 |
69.4 |
18.4 |
|
2301 |
38-67 |
551 |
3.0 |
2 5 |
94.5 |
|
|
67-200 |
265 |
0.1 |
0.5 |
99.3 |
|
|
0-78 |
141 |
10.9 |
60.0 |
29.1 |
|
|
78-105 |
188 |
18.5 |
38.5 |
43.1 |
|
3732 |
105-128 |
925 |
0.6 |
0.4 |
99.0 |
|
|
128-200 |
1426 |
0.9 |
0.3 |
98.8 |
|
|
0-34 |
350 |
26.7 |
60.0 |
13.3 |
|
|
34-80 |
262 |
32.3 |
58.1 |
9.7 |
|
3749 |
80-115 |
190 |
31.8 |
50.0 |
18.2 |
|
|
115-200 |
113 |
6.7 |
46.7 |
46.7 |
|
|
0-50 |
176 |
36.1 |
25.0 |
38.9 |
|
|
50-90 |
194 |
20.6 |
3.2 |
76.2 |
|
4059 |
90-115 |
233 |
17.7 |
5.1 |
77.2 |
|
|
115-175 |
169 |
13.9 |
41.7 |
44.4 |
|
|
175-200 |
106 |
13.2 |
47.2 |
39.6 |
|
|
0-40 |
378 |
3.9 |
62.3 |
33.8 |
|
|
40-95 |
307 |
9.1 |
72.7 |
18.2 |
|
4454 |
95-152 |
284 |
15.4 |
74.4 |
10.3 |
|
|
152-200 |
262 |
12.0 |
70.0 |
18.0 |
a. Expressed as percent of total active inorganic P.
Figure 2. Distribution of organic P within the soil profiles of some of the Vertisols.

Organic phosphorus
Organic P content in the surface soils ranged between 99 and 367 ppm (Table 4). In general, organic P content decreased with depth, as shown in Figure 2. Organic P values tended to vary according to the organic matter contents of the profiles, indicating a close relationship between the two variables. According to Tekalign and Haque (unpublished data 1), a highly significant correlation was observed between organic P and organic matter in 32 Ethiopian surface soils. Such a positive relationship between organic P and organic matter has also been reported by other workers (Black and Goring, 1953; Uzu et al, 1975).
The organic C: organic P ratio (Table 3), an index of the mineralization capacity of organic P. was below 200 in the profile samples of all soils except the surface sample of Shola (soil 1615), indicating a possible rapid turnover rate for organic P. Similar low values were also observed by Piccolo and Gobena Huluka (1986) in some Ethiopian soils. Under tropical conditions, organic P is readily mineralised into inorganic P (Tisdale and Nelson, 1966) and can thus be an additional P source to plants. According to Tekalign and Haque (unpublished data 1), organic P in 32 Ethiopian surface soils was found to constitute about 41% of the total P content. According to Ahmad (1986), organic P content is estimated to be as high as 40-50% of the total P in the Vertisols of the Ethiopian highlands.
There was a common tendency of the C:P ratio to decrease with depth. Similar trends were reported in some tropical soils by Bornemisza (1966), but this was not confirmed by Piccolo and Gobena Huluka (1986) in their studies on some Ethiopian soils.
Active phosphorus fractions
The amount and distribution of the various active inorganic P fractions (the phosphates associated with Ca (Ca-P), Fe (Fe-P) and Al (Al-P)) are shown in Tables 4 and 5. The percentage distribution of the three active P forms varied among the eight soil profiles, reflecting the different conditions in which they were formed. Ca-P was more abundant at lower depths in soils 1614, 2301 and 3732 (Table 5). This can be explained on the basis of the occurrence of less weathered parent material in the profiles. In addition, because Ca is the dominant cation in all the profiles (Kamara and Haque, 1987), added P might be transformed to Ca-P and moved further down due to the seasonal physical movement of the soil.
For the surface samples of the profiles, the distribution of the active P forms was in the order Fe-P > Ca-P > Al-P in 10 out of the 15 surface soils (Table 4). However, when the values within each profile for all the profiles were considered, the order was mixed (Table 5). Previous studies on different Ethiopian surface soils show that the active inorganic P fractions were found in the order Ca-P > Fe-P > Al-P (Desta Beyene, 1982; Tekalign and Haque, unpublished data 2). On the other hand, Piccolo and Gobena Huluka (1986), working on 7 Ethiopian soils (2 Pellic and 1 Chromic Vertisols, 2 Eutric Nitosols, 1 Dystic Cambisol and 1 Calcic Fluvisol) found that the relative abundance of the inorganic P forms in the profiles was in the order: Fe-P > Ca-P > reductant soluble P.
In the present study, the abundance of Fe-P in the poorly drained surface samples is supported by the general feet that under flooded conditions, Fe-P more than the other fractions is the source of available P (Uzu et al, 1975). The profile average of Fe-P is higher in soils from Shola (soil 1615) than in soils from Debre Birhan (soils 2299 and 2301). This is expected since Debre Birhan soils have among the highest contents of oxides of Fe and Al, as shown in Table 2. The contents of both Al-P and Fe-P at the lowest depth were almost invariably lower than the values at the surface, the exceptions being soils 4059 (for Fe-P) and 2299 (for Al-P).
Available phosphorus
Next to N. P is the most limiting nutrient in Vertisols (Finck and Venkateswarlu, 1982) and this holds true for Ethiopian soils. Available P contents, determined by the method of Olsen et al (1954), and the two methods of Bray and Kurtz (1945), are shown in Table 4. The profile distribution of Bray II available P is also shown in Table 3 for some of the profiles. Available P was low by the three methods in most of the surface soils. Using the Olsen method, which is often regarded as the most appropriate for Ethiopian soils (Tekalign and Haque, unpublished data 2), the maximum P content was observed in soil 3669 and the minimum in soil 1615. Interestingly, soil 1615 has also the lowest total P content after soil 3732.
Higher values of Bray II extractable P were observed at lower depths than at the surface for each of the profiles, as shown in Table 3. This may be due to the abundance at lower depths of Ca-P and the dissolution of Ca-P by the Bray II extractant. Similar trends were also observed by Piccolo and Gobena Huluka (1986) in their P studies of 7 Ethiopian soils.
The status of available P in soils is normally related to the different active inorganic P forms Al-P Fe-P, and Ca-P). In a previous study in which 32 Ethiopian surface soils (including 7 of the 15 Vertisols in this study) were considered, Tekalign and Haque (unpublished data 1) found that available P estimated by the Bray I, Bray II and Olsen methods was better correlated with Al-P and Ca-P. This was later supported by a further study (Tekalign and Haque, unpublished data 2) in which Al-P and Ca-P were also the P forms that correlated best with plant P uptake. Based on these findings for the 15 Vertisols included in this study, the low Al-P and Ca-P contents reported in the surface soils are indicative of the limited capacity of the inorganic forms to act as a labile pool to supply available P to the plants.
In his survey of nutrient availability in 350 surface soil samples in the Shewa region of Ethiopia, Pulschen (1987) found that the mean Olsen-extractable P in 165 Vertisols or soils with vertic properties was 11.6 ppm-less than that in light soils (16.9 ppm) or reddish brown soils (13.9 ppm).
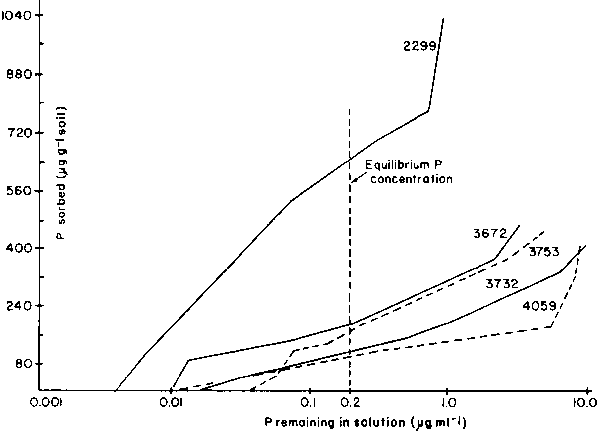
Phosphorus sorption
The data from the P sorption experiment are plotted in Figure 3 for low, medium, and high P sorbing soils. In all eases, the sorption and equilibrium P concentration in solution continued to increase with higher rates of P additions There are three categories of P sorption isotherms based upon the quantity of P sorbed (Table 4): low P fixing soils, such as soil 4059; medium to high P fixing soils, such as soils 3753 and 3672; and very high P fixing soils, such as soil 2299.
Phosphorus sorption was positively correlated with contents of organic matter, (r² = 0.364 (P<0.05)) extractable Fe (r² = 0.623 (P<0.01)) and Al (r² = 0.660 (P<0.001)). The correlation of fixed P with clay content or pH was poor; in addition, there was no correlation between fixed P and pH.
Based upon the classification by Sanchez and Uehara (1980) of soils in terms of P sorption, it follows that about 70% of the Vertisols included in this study are high P fixing soils. This is in close agreement with previous reports (Tekalign and Haque, 1987) which showed that about 65% of 32 different Ethiopian surface soils were in the higher P sorption range.
Figure 3. P sorption characteristics of some of the Vertisols.
Results show a wide range of differences in P status of the soil samples studied. The majority of the soils are low in available P; about 70% of the soil samples are deficient in P. Phosphorus fraction results show low levels of the available forms. Phosphorus sorption studies indicate high sorption capacity of the soils. Phosphorus sorption is mainly controlled by content of Fe and Al oxides. More studies are needed to understand the P status of other Ethiopian Vertisols and related soils.
Ahmad N. 1986. Soil and agronomic factors influencing fodder production in the Ethiopian highlands of East Africa. PSD Working paper No. B4. International Livestock Centre for Africa (ILCA), Addis Ababa, Ethiopia. pp. 58.
Berhanu Debele. 1985. The Vertisols of Ethiopia: their properties, classification and management. In: Fifth Meeting of the Eastern African Sub-Committee for Soil Correlation and Land Evaluation. Wad Medani, Sudan, 5-10 December 1983. World Soil Resources Reports No. 56. FAO (Food and Agriculture Organization), Rome. pp. 31-54.
Black C A and Goring C I A. 1953. Organic phosphorus. Agronomy 4:123-152.
Bornemisza E. 1966. Elfosforo Organico en suels tropicales. Turialba 16:33-38.
Bouyoucos G J. 1951. A recalibration of the hydrometer methods for making mechanical analysis of soils. Agronomy Journal 43: 434-438.
Bray H R and Kurtz L T. 1945. Determination of total organic and available forms of phosphorus in soil. Soil Science 59:39-46.
Chang S C and Jackson M L. 1957. Fractionation of soil phosphorus. Soil Science 84: 133-144.
Desta Beyene. 1982. Diagnosis of phosphorus deficiency in Ethiopian soils. Soil Science Bulletin No: 3. Institute of Agricultural Research, Addis Ababa, Ethiopia. 18 pp.
Dudal R. 1965. Dark clay soils of tropical and sub-tropical regions. Agricultural Development Report 83. FAO (Food and Agriculture Organization), Rome. 161 pp.
Finck A and Venkateswarlu J. 1982. Chemical properties and management of Vertisols. In: Vertisols and rice soils of the tropics. Twelfth International Congress of Soil Science, New Delhi, India, 8-16 February 1982. Indian Society of Soil Science, New Delhi, India. pp. 61-79.
Fox R L and Kamprath E J. 1970. Phosphate sorption isotherms for evaluating the phosphorus requirements of soils. Soil Science Society of America Proceedings 35: 902-907.
Hubble G D. 1984. The cracking clay soils: definition, distribution, nature, genesis and use. In: J W McGarity, H E Hoult and H B So (eds), The properties and utilization of cracking clay soils. Reviews in Rural Science No. 5. University of New England, Armidale, NSW, Australia. pp. 3-13.
Jackson M L. 1964. Soil chemical analysis. Englewood Cliffs, Prentice Hall, New York.
Jutzi S and Haque I. 1985. Management of deep clay soils (Vertisols) in sub-Saharan Africa. ILCA/ICRISAT research, training and outreach project. ILCA (International Livestock Centre for Africa). 23 pp.
Kamara C S and Haque I. 1987. Characteristic of Vertisols at ILCA research and outreach sites in Ethiopia. PSD Working Document No. B5. International Livestock Centre for Africa (ILCA), Addis Ababa, Ethiopia.
Legg J O and Black C A. 1955. Determination of organic phosphorus in soils: II. Ignition method. Soil Science Society of America Proceedings 19:139-143.
Mehra O P and Jackson M L. 1960. Iron oxide removal from soils and clays by dithionite - citrate system buffered with sodium bicarbonate. Clays and Clay Minerals 7:317-327.
Murphy J and Riley J P. 1962. A modified single solution method for the determination of phosphorus in natural waters. Analytica Chimica Acta 27:31-36.
Olsen R A and Engelstad O P. 1972. Soil phosphorus and sulfur. In: Soils of the humid tropics. National Academy of Sciences, Washington DC. pp. 82-101.
Olsen S R. Cole C V, Watanabe F S and Dean L A. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939. Washington DC.
Peterson G W and Corey R B. 1966. A modified Chang and Jackson procedure for routine fractionation of inorganic soil phosphates. Soil Science Society of America Proceedings 30:563-565.
Piccolo A and Gobena Huluka. 1986. Phosphorus status of some Ethiopian soils. Tropical Agriculture 63:137-142.
Pulschen L. 1987. Terminal report for the period May 1983-April 1987. Soils and Plant Nutrition Section, Agricultural Center, Debre Zeit, Ethiopia. 31 pp.
Sanchez P and Uehara G. 1980. Management considerations for acid soils with high phosphorus fixation capacity. In: The role of phosphorus in agriculture. Symposium proceedings. 1-3 June 1976. ASA, CSSA, SSSA, Madison, Wisconsin, USA. pp. 471-514.
Tekalign Mamo and Haque I. 1987. Phosphorus status of some Ethiopian soils. I. Sorption characteristics. Plant and Soil 102:261266.
Tisdale S L and Nelson W L. 1966. Soil fertility and fertilizers. MacMillan, New York.
Uzu F 0, Juo A S R and Fayemi A A A. 1975. Forms of phosphorus in some important agricultural soils of Nigeria. Soil Science 120:212-218.
Walkley A and Black I A. 1934. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Science 37:29-38.