

©Shutterstock/Vadim Petrakov/
4.5 Cell-based food production
While the world begins to understand the importance of transforming the current agrifood systems to be more sustainable and environmentally conscious, there is also an increasing consumer demand for animal-based food products worldwide (FAO, 2018). The intensification of animal production may contrast with sustainability objectives, resulting in trade-offs in various environmental aspects, food security and animal welfare (FAO, 2020; Henchion et al., 2021; OECD and FAO, 2021). New technology presents a potential alternative: the production of land and aquatic animals without requiring large-scale farming and slaughtering.
In 1932, Winston Churchill stated: “We shall escape the absurdity of growing a whole chicken in order to eat the breast or wing, by growing these parts separately under a suitable medium” (Churchill, 1932). After decades of research and development, the technology has now matured, and his idea has become a reality. The production can be done via in vitro cultivation of animal cells and then processed into products whose composition can be equivalent to conventional animal products without needing the whole animal (Kadim et al., 2015; Post, 2014).
Since the initial studies in the early 2000s, cell-based food production methodologies have been well characterized, meaning they are now ready to move from laboratories to production facilities. In 2013, the first beef burger produced through this technology was presented to the world (Jha, 2013). In December 2020, the first cell-based chicken nuggets were approved by a competent authority in Singapore. As of November 2021, there are at least 76 companies developing similar products around the world (Byrne, 2021). Many types of products and commodities such as various types of meat, poultry, fish, aquatic products, dairy and eggs are in the pipeline for future commercialization.
Terminology and definitions
Various terms are currently in use (Box 9), as yet there is no internationally harmonized terminology to indicate this type of food product or the production process (Ong, Choudhury and Naing, 2020). For example, some people call meat analogues “cultured”, “cell-based” or “cultivated” meat. Product marketers may call it “animal-free”, “clean” or “slaughter-free” meat. For the purpose of the present brief, and without setting a precedence, the term “cell-based” is used. Some may identify the whole technology as “cellular agriculture” or “cell-culturing”. The lack of clear definitions for these terms creates the potential for confusion. National authorities will be most effective if the terminology they use is 1) transparently representative of the products; 2) informative for food labelling, clearly communicating to consumers that the products produced through the new technology are different from the conventional products with which they may already be familiar, but also contain the same potential allergens; and 3) neither disparaging nor generating consumer reactions (Hallman and Hallman, 2020).
Box 9. Some modifiers or adjectives used as terminology for cell-based food products
- animal-free
- artificial
- cell-based
- cell-cultured
- cellular
- clean
- cruelty-free
- cultivated
- cultured
- in vitro
- lab-grown
- slaughter-free
- synthetic
- test tube
- vat-grown
What are the food safety implications to be considered?
Production overview and hazard/concern mapping
Food safety is one of the foremost concerns when new technology is applied to food production processes. Within the risk analysis paradigm, the first step of safety assessment is hazard identification, which can be conducted following the production steps. For cell-based food production, the methodologies and production steps can greatly vary depending on the company, the desired final product, manufacturing facilities and equipment. To illustrate the indicative food safety hazard identification process, a generic overview of production steps is presented in Box 10, followed by a generic map of potential hazards/concerns (Table 4).
Box 10. A generic production overview of cell-based food products
- Cell selection from the source animal
- Production: The cells selected in step 1 are allowed to multiply in bioreactors; cells may be anchored to microcarriers or a scaffold to organize tissues in a 3D structure.
- Cell preparation
- Cell proliferation
- Cell differentiation
- Harvesting of the product
- Food processing: The harvested products may be processed further to shape it in desired forms and/or be combined with other ingredients for commercialization
Table 4. A generic map of potential hazards/concerns in cell-based food production processes
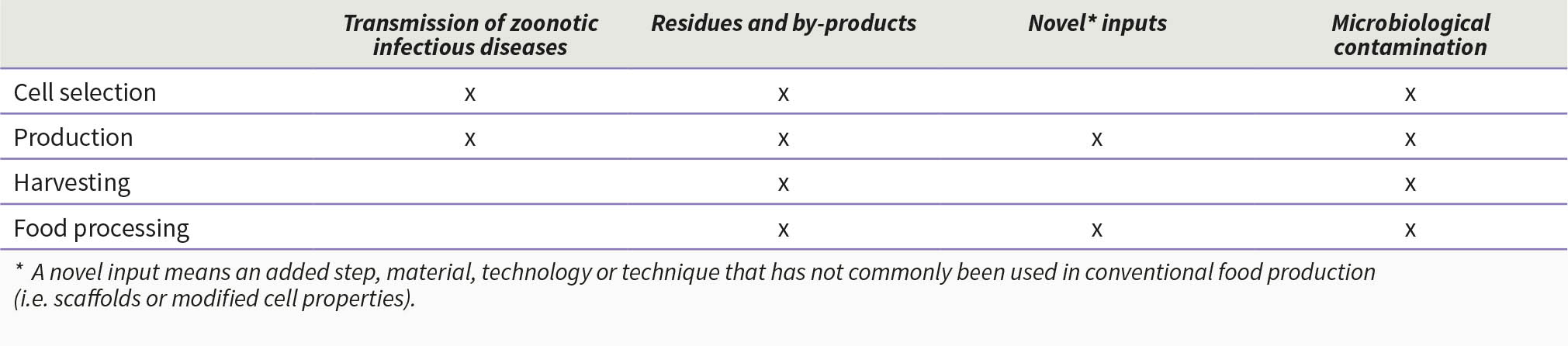
Potential food safety hazards/concerns
Source cell lines: The desired starting cell lines are often sourced from a live or slaughtered animal of choice followed by cell isolation. A common alternative is to use induced pluripotent stem cells (iPSCs), reprogrammed adult cells that can differentiate into any type of cells (Takahashi and Yamanaka, 2006). Although iPSCs have been well studied in mice since their discovery, the differentiation protocols for various livestock animal cells such as chicken remain elusive (Post et al., 2020).
The chance of infectious zoonotic and foodborne disease occurrence is considerably reduced when compared to conventional livestock production (Treich, 2021), but major considerations must be given to the use of animal serum in the culture media, which may introduce pathogens including virus, bacteria, parasites as well as prions (Hadi and Brightwell, 2021; Ong et al. 2021). However, early detection of cell infections via careful monitoring can greatly limit such hazards. Also, as it is for any food production processes, following good hygiene practices (GHP) throughout the whole production process is critical.
The entirety of cell-based food production can be done in a well-controlled environment without the risk of contamination from faeces or external sources (Chriki and Hocquette, 2020). However, the application of antibiotics during some of the production steps may still be conducted. Consequently, residues may remain in the final product as antimicrobial residue (Agmas and Adugna, 2018).
Components of the growth medium: Animal-serum based culture media, especially those with fetal bovine serum (FBS), are currently the most common option (Hadi and Brightwell, 2021; Post, 2012; Post et al., 2020); and they may present a higher risk of microbiological contamination (Chriki and Hocquette, 2020). Such hazards can be managed and controlled by monitoring for key pathogens appropriately (Specht et al., 2018). Moreover, there has been a substantial effort in developing animal serum-free media to overcome concerns surrounding FBS, and there are currently at least 100 different media formulations available (Andreassen et al., 2020).
Adherent surfaces: For cells to increase in size and to generate muscle fibres, they are attached to 3D scaffolds, which physically exercise the cells. Scaffolds can be either synthetic or made up of edible materials, the latter may be preferable as they do not have to be removed from the final product (Allan, Ellis and De Bank, 2021; Campuzano, Mogilever and Pelling, 2020; MacQueen et al., 2019). Most biomaterials used as scaffolds in cell-based food production are not known to cause allergic reactions upon consumption. Careful attention needs to be paid to ensure materials derived from known sources of allergenicity are not inadvertently introduced. For instance, chitin or chitosan may trigger allergic responses in individuals who are also allergic to crustaceans.
Changes in physico-chemical properties: To obtain exponential cell growth and optimum cell density, the initial cell lines are constantly sub-cultured (Masters and Stacey, 2007). As in all cell lines that are allowed to propagate over many generations, there can be a risk that genetic or epigenetic drift may occur and this needs to be suitably monitored. (Ong et al., 2021).
Cryoprotectants: Cryoprotectants such as inulin and sorbitol can be used for cell storage (Elliot et al., 2017). Care must be taken that no carry-over into the final product occurs at concentrations that may cause a risk for consumers (MacDonald and Lanier, 1997; Savini et al., 2010).
Microbiological contamination throughout the process: As with all food processing and fermentation techniques, cleanliness of operations, ongoing monitoring and strict adherence to GHP and GMP are critical to avoid microbiological contamination, which may occur at any step of the production process. Application of the hazard analysis (and) critical control point (HACCP) system is also considered to be effective.
End-product food safety assessment
FAO, together with the World Health Organization (WHO), provides scientific advice to the Codex Alimentarius, the international food standard setting body, according to established principles and guidelines for the risk assessment of individual substances such as chemical additives, residues and contaminants (FAO, 2021a), microbiological risk assessment (FAO, 2021b), and whole food safety assessment (FAO and WHO, 2011). Molecular characterization, biochemical/physical analysis, assessment on toxicity and allergenicity, and nutritional composition analysis are the main elements of the generic whole food safety assessment (FAO and WHO, 2008). Experts suggest such standardized principles and methodologies are applicable to conduct end-product food safety assessment of cell-based food. As of today, all risk assessments of whole food items are performed on a case-by-case basis, and no consensus has yet emerged as to when cell-based food products require a separate risk assessment.
Novelty and food safety considerations
Ong et al. (2021) has listed the key areas of research to enhance the food safety assurance of cell-based food products and stated that it is important to focus on the products’ novelty. Despite the potential knowledge gaps and uncertainties that may be present, most identified hazards and concerns are unlikely to be new, thus prioritizing any novelty and differences in the process and the products is key (Ong et al., 2021).
What are the drivers and other key considerations?
Is it meat?
“Cell-culturing” technology can use both plant and animal cells as a source, and it can also lead to the production of acellular products such as milk, proteins or fats (Rischer, Szilvay and Oksman-Caldentey, 2020). While plant-based meat alternatives would not be categorized as meat, it is not yet clear whether this is also true for animal cell-based food products. Furthermore, if cell-based meat is categorized as meat and/or includes “meat” in its name, it may have various implications for relevant existing regulations for safety and quality assurance and labelling.
Who should be in charge?
The glossary of the World Organisation for Animal Health (OIE) states that meat “means all edible parts of an animal” (OIE, 2021), but an animal does not necessarily have to be involved in cell-based food production. The chosen nomenclature may therefore define who will oversee the management of cell-based food products at the regulatory level. Depending on the existing national regulatory frameworks and the categorization choice, cell-based food products can fall under the regulations of 1) meat/livestock (or other commodity-related sector), 2) alternative proteins, 3) novel foods, 4) food safety or 5) any combinations of the above.
Sustainability and environment
While less land use is expected for cell-based food production when compared to conventional livestock farming, this comparison is not straightforward as livestock farming also plays important environmental roles such as maintaining soil carbon content and soil fertility (Chriki and Hocquette, 2020). According to Mattick (2018), cell-based food production may also have a reduced potential for eutrophication, similar to conventional poultry production, but lower than beef or pork (Table 5).
Table 5. Comparison of estimated environmental impacts of producing 1 kilogram of meat (conventional and cell-based) products in the United States of America
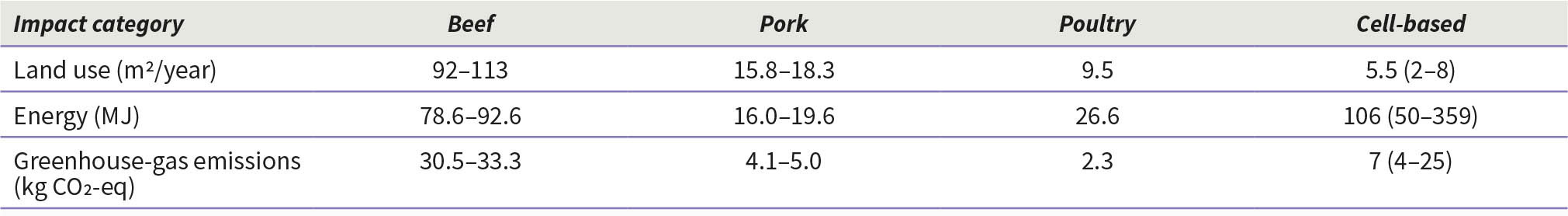
The potential advantage of cell-based meat over livestock in terms of greenhouse gas emissions is not clear. Methane (CH4) emissions are the primary concern with ruminants, in addition to carbon dioxide (CO2) and nitrous oxide (N2O). On the contrary, CO2 is the main greenhouse gas associated with cell-based food production due to high fossil energy use. Lynch and Pierrehumbert (2019) concluded through their modelling studies that cattle farming may be a better option than cell-based meat production due to the high fossil-fuel energy use of the latter while assuming that current consumption patterns of meat are maintained. Mattick et al. (2018) suggested that cell-based meat could involve some trade-offs, with high energy use leading to cell-based meat having potentially greater global warming impacts than pork or poultry, but lower than beef, while retaining possible gains in land use. Smetana et al. (2015) noted that among cell-based meat, the various protein alternatives (plant-based, mycoprotein-based, dairy-based) and chicken, cell-based meat had the highest environmental impact due to its high energy requirements but had lower land use and eutrophication potential than others. This may lead national authorities to consider, in addition to the definite need for food safety assurance, the need for overall environmental impact assessment and monitoring.

©UPSIDE Foods/David Kay
Food and nutrition security
Cell-based food must be produced indoors without being disrupted by extreme climate conditions; therefore, some developers claim that this may contribute to food security. Also, animal-derived products (meat, poultry, dairy, eggs, fish and aquatic food products) are a significant source of protein. Seeking more efficient ways to produce such proteins may help ensure nutrition security. Cell-based food production is presented by some as an option for those who want to act responsibly without altering their diets and cultural norms (Chikri and Hocquette, 2020; Shapiro, 2018). In addition, it is suggested that some countries may find the technology attractive for rendering their food supply more self-sufficient through cell-based production, without having to expand and intensify their current livestock and/or aquaculture production.
Animal welfare
Some developers substantiate the importance of this technology with the claim that it will drastically improve animal welfare (Bhat, Kumar and Fayaz, 2015) as the overall number of livestock raised and slaughtered are expected to be significantly reduced (Schaefer and Savulescu, 2014). However, as the first step is generally to conduct biopsies on animals to collect the cells, some may still have concerns over animal welfare issues since some animals would still need to be raised (Alvaro, 2019) and potentially slaughtered.
Food loss
From a food loss perspective, carcass utilization has been a challenging issue in conventional livestock farming. There are companies such as gelatine, pet food and fish feed manufacturers, that do utilize byproducts from livestock and therefore help to reduce food loss. Cell-based food production can provide the means of producing meat that greatly contributes to resolving issues related to carcass utilization (Stephens et al., 2018). However, the environmental impacts that may occur if other products of livestock farming, such as leather and wool, are produced separately and the economic impacts on such industries have not been explored (Mattick, Landis and Allenby, 2015).
Aquatic cell-based food products
While aquatic cell-based food production may open the door for aquatic resource-poor countries, this specific sector has an additional terminology-related consideration. Aquaculture products are usually referred to as “farmed” or “cultured” fish/seafood in order to be distinguished from wild-catches. Therefore, the terms used for cell-based food production of aquatic products may need different words to clearly differentiate aquaculture products from cell-based aquatic products (Hallman and Hallman, 2020).
Ethics, religion, lifestyle and philosophy
As the technology requires significantly fewer animals than conventional livestock farming, cell-based food products may be attractive to those who follow a vegetarian or vegan lifestyle. Any ethical issues raised with regards to cell-based food production will need due consideration. In addition, questions may be asked about whether such products can be considered Kosher, Halal and so forth keeping with the respective religions, values and/or traditions (Hamdan et al., 2018; Krautwirth, 2018).
Consumer perceptions
Not every consumer is necessarily knowledgeable of the science behind cell-based food production, and the terminology will eventually affect the meaning and connotations attributed to cell-based food products (Bryant and Barnett, 2019; Byrant et al., 2019). Learning from past technology-driven food production, it is extremely important for the competent authorities to understand consumer perceptions in the local context and to start inclusive and transparent dialogue with them at the earliest stage possible (Nucci and Hallman, 2015).
Production costs and product prices
The first cell-based beef hamburger was created at a cost of USD 375 000 in 2013 (Kupferschmidt, 2013) and the first cell-based chicken nugget for USD 50 in 2019 (Corbyn, 2020). The production costs for cell-based meat have fallen but remain expensive for large-scale retail purposes. The growth media currently make up a bulk of the total production costs for cell-based meat (Choudhury, Tseng and Swartz, 2020; Swartz, 2021). In addition, substituting fossil fuel-based energy with renewable energy sources, maintaining adequate oxygen supply, wastewater treatment, transportation across the globe as well as labour expenses may also drive up the cost of the final product (Mattick, 2018; Risner et al., 2020). However, cell-based food products have the potential to be sold at USD 5.66 per kg by 2030, which is cheaper than some of the conventional meat currently on the market (Swartz, 2021).
Regulations for commercialization
If cell-based food products fall in a category that requires food safety assessments according to the existing regulatory frameworks, it is a responsibility of the food safety competent authorities to set up the procedures for such assessments. Also, if consumers demand special labelling, it is the relevant authorities’ responsibility to establish a clear policy. Labelling is usually not a straightforward issue to manage, as it almost always requires the quantification of the ingredients/products. Thus, in this case, the policy will need to set a threshold of how much of the food has been produced through cell-based techniques for the purpose of labelling.
International trade
It is always important to consider the case of asynchronistic regulatory approvals. Some countries might not even require regulatory approvals, and some might struggle in establishing the approval process with limited technical capacities. However, the reality is that once a cell-based food product has been approved in one country, it is only a matter of time for that product to travel to another country where regulatory frameworks may be different. For this reason, it is important to have inclusive global dialogues at an early stage so that the sharing of information and experiences can benefit many low- and middle-income countries (LMICs). FAO has begun several initiatives to provide scientific advice on the food safety considerations of cell-based food products (Box 11).
Box 11. FAO initiatives for cell-based food production
To provide timely and sound scientific advice on food safety aspects of cell-based food production, the following activities are ongoing.
- Three preliminary technical papers on:
- – nomenclature;
- – existing regulatory frameworks; and
- – existing production processes for food safety hazard identification.
- Consultations with the relevant international agencies and bodies (i.e. WHO, OIE, OECD, Codex), national food safety competent authorities, academia, research institutes and the private sector
- Case studies from two countries
- Global expert consultation (to be organized in late 2022 or 2023)
What is the way forward?
As described in the food safety consideration section, the majority of the potential hazards in this technology is not new. Thus, it is important to learn from various past experiences and consider effective application of the risk analysis paradigm (Ong et al., 2021). In adopting several established safety assessment/evaluation methodologies in a range of disciplinary fields such as pharmaceuticals and food biotechnologies including both conventional and modern technologies, various hazards can be systematically identified, and relevant safety assessments can be appropriately conducted. There are also many risk-mitigating tools available in the area of food safety, such as good practices (GHP, GMP, GCCP and HACCP) and general principles and methodologies for the end-product whole food safety assessment (FAO and WHO, 2009). While there are many existing tools that can be useful for the safety assessment, additional steps for the safety assessment might be required for some particularly novel processes or products. Therefore, with cell-based food products, it is important to focus on the significant differences from existing foods so that effective methodologies to assess the safety of all elements can be established.
Many countries have not yet experienced an urgent need to conduct food safety assessments of cell-based food products. However, preparedness is key; and it is important for the competent authorities to start dialogues with various stakeholders including consumers, private sector, civil society, partner agencies and policy makers. Experts have emphasized the importance of securing inclusiveness and transparency, while preparing for necessary regulatory actions (FAO and WHO, 2016). For LMICs, it is also important to initiate the assessment of technical capacity for safety assurance of cell-based food products as they may benefit from having dialogues with other countries and international organizations to learn from their experiences and to obtain technical assistance. Engaging in the relevant global discussions is recommended for all countries, as shared information and data can only contribute to the global good, without duplication of efforts.
Food safety is a joint responsibility. Active and transparent communications through public and private collaboration are crucial not only to better prepare the industries and governments, but to maximize the effectiveness of their safety assurance programmes. Competent authorities’ clear food safety guidelines for the private sector would enable and promote the “safety by design” approach to jointly aim at assuring food safety of cell-based food production
